Background
Chemical burn is an acute eye injury occurring in home, industrial and military environments, accounting for 20% of all eye injuries. Severe chemical burn is extremely harmful to the eye, which can lead to eyelid skin deformity, corneal opacity and neovascularization, anterior chamber inflammation and exudation, cataracts, and even retinal and optic nerve damage. Current treatments focus on removing chemicals by rinsing early in the burn and reducing subsequent inflammation, although chemical burns can still cause damage to the eye, leading to permanent vision loss and blindness.
Previous studies have found that oxygen plays a key role in maintaining tissue vitality, and this finding has been applied in many ways, such as the use of hyperbaric oxygen to treat diabetic wounds, and the delivery of oxygen throughout the body through nasal cannula or mask to improve the prognosis of chemical damage to the eyes in animals and humans, but the therapeutic effect of this therapy is not obvious. And it can only be done in the presence of special equipment.
Perfluorocarbons (PFCs) are inert chemicals with good biocompatibility and oxygen dissolving ability. At room temperature, the solubility of oxygen (O2) is above 40% in PFC, 2.5% in plasma, and 20% in whole blood. The oxygen binding capacity of PFC is related to the low polarization of fluorine, and the solubility of the gas in PFC is proportional to the partial pressure of the gas. Perfluorodecahydronaphthalene (PFD) is a member of the PFFC family and can be used both as an oxygen carrier and as an intraoperative adjunct for reattachment of detached retina. Pfd-based oxygen emulsions have been shown to improve wound healing in second-degree burns on human and pig skin.
We report the development of a PFD-based ophthalmic supersaturated oxygen emulsion (SSOE) and evaluate its safety and efficacy in alleviating acute eye injury in a mouse model of alkali burn.
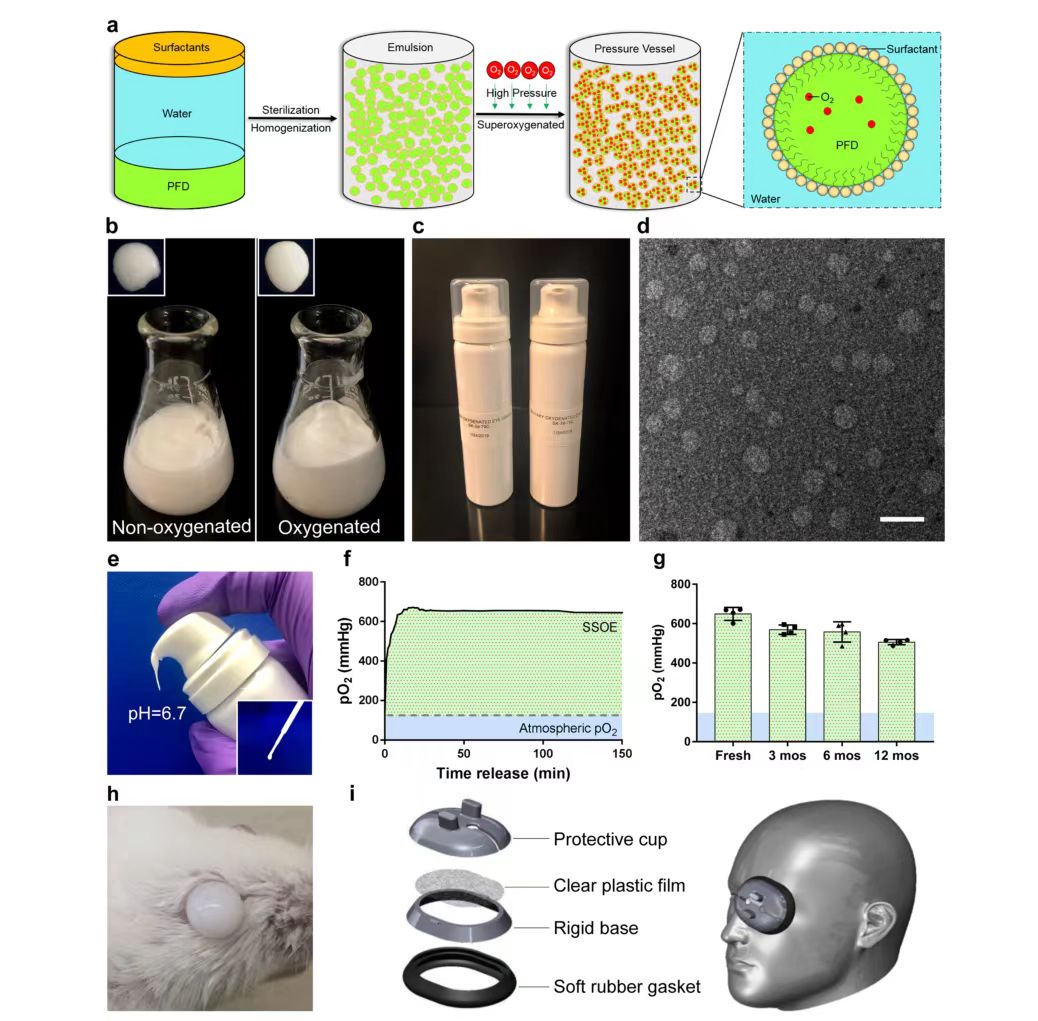
Among all PFCs, perfluoronaphthalene (PFD) has a high oxygen carrying capacity, at room temperature, every 100 mL of PFD can dissolve 49 mL of oxygen, so it is widely used in medical applications, SSOE is formulated from perfluoronaphthalene. Because PFD is hydrophobic, two surfactants, Phospholipon 90H and Polawax (fixed ratio 5.0:1.8), were used to emulsify PFD. In short, 25% (w/v) PFD is homogenized with Phospholipon 90H and Polawax and water, and then the emulsified nanoparticles are supersaturated with medical grade oxygen in a high-pressure reaction vessel. Oxygen-saturated emulsions have a more uniform and reflective appearance than non-oxygen-saturated emulsions (Figure 1b). Finally, the oxygen-saturated SSOE is stored in a small pressurized dispensing tank (Figure 1c). When needed, the emulsion can be dispensed directly from the tank without additional equipment or operation (Figure 1e). A disposable pipette can also be used to transfer the emulsion (Figure 1e, illustration). The emulsion has a pH of 6.7, which is within the safe range for ophthalmic use, and the oxygen partial pressure (pO2) within the emulsion can peak at 600 mmHg within a few minutes after emulsion distribution and persist above 600 mmHg for at least 2.5 hours (Figure 1f), which is four times that of atmospheric pO2 (160 mmHg), due to its inherent viscosity. SSOE remains on the ocular surface after application, as shown in Figure 1h. Unpacked emulsions stored in normal environments at room temperature for up to 1 year showed a slight decrease in pO2 compared to newly prepared emulsions for long-term storage, but could still remain above 500 mmHg when distributed after 1 year of storage (Figure 1g). The structure of the SSOE particles was observed under a transmission electron microscope (Figure 1d), with most particles ranging in diameter from 50 to 300 nm. Figure 1i shows an accessory device consisting of a protective cap, clear plastic film, rigid base, and soft rubber washer designed by the researchers to increase the loading capacity and improve the application of SSOE in the human body.
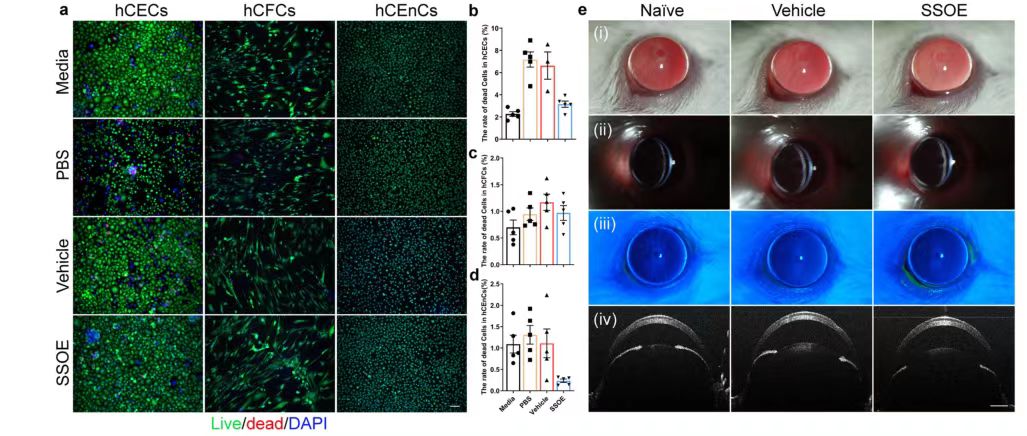
To investigate the safety of SSOE in ophthalmology, researchers used cultured human corneal epithelial cells (hCECs), corneal stromal fibroblasts (hCFCs), and corneal endothelial cells (hCEnCs). The number of live/dead cells after 1 hour of culture-medium control, PBS control, anaerobic emulsion (carrier) control or SSOE administration was compared, and the results showed: In the study of hCECs and hCFCs after SSOE treatment, the number of cell death increased compared with the medium treatment group, but decreased significantly compared with the other two groups. In the study of hCEnCs, the number of cell death after SSOE treatment was significantly lower than that in the other three groups.
The researchers also conducted a modified Draize trial in mice, where SSOE or a carrier control was applied topically to mouse eyes for 1 hour, then rinsed, and the mice were observed continuously for 1 week. Slit-lamp photographs taken 4 hours after administration (FIG. 2e) did not show any eyelid irritation or redness, corneal opacity, or edema. Abnormalities such as intraocular inflammation, anterior chamber exudation, or decreased lens transparency were observed. In addition, corneal fluorescein staining showed that the epithelial barrier function was intact and the corneal epithelium was free of defects (Figure 2e ii). Optical coherence Tomography (OCT) imaging did not show changes in corneal thickness or anterior chamber depth, nor did it show any structural changes in the eye (Figure 2e iv). These results indicate that SSOE is biocompatible with corneal cells and is safe in vivo for mouse eyes.
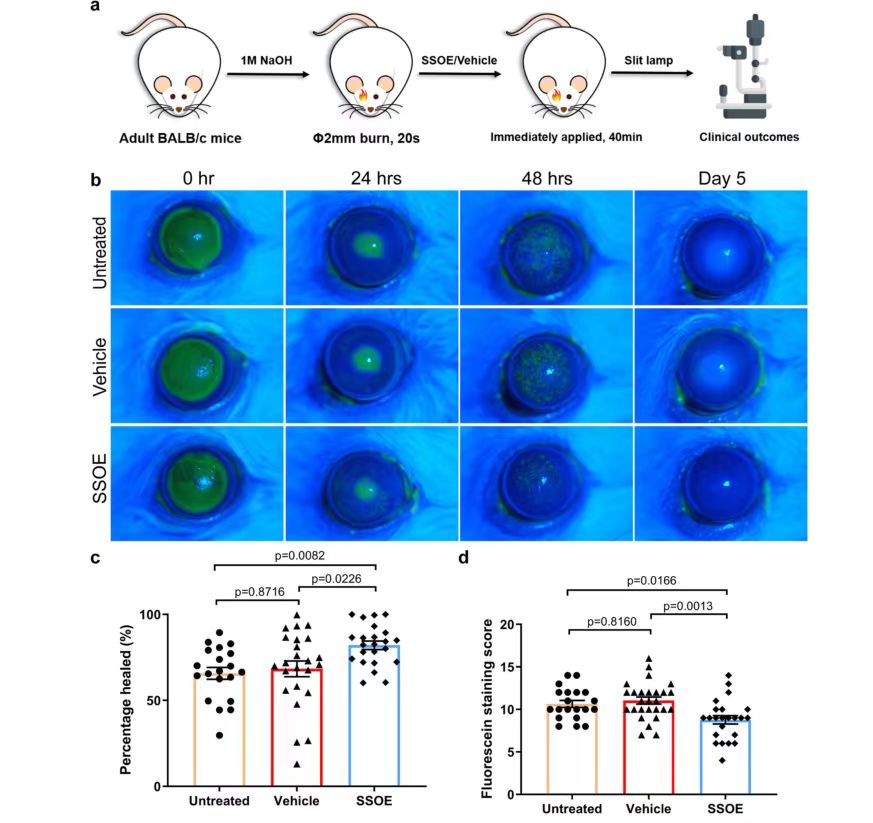
To evaluate the efficacy of SSOE in the treatment of acute alkali burns, burn corneas with sodium hydroxide (NaOH) in adult BALB/c mice, followed by extensive flushing, and wash off the emulsion after applying SSOE or carrier control to the ocular surface for 40 minutes while the mice were under anesthesia. Antibiotic eye cream was also used in all groups to prevent infection. Mice with alkali burned corneas but not treated with SSOE or carrier were also included in the untreated control group for clinical evaluation of eye changes and photographed with slit-lamp (Figure 3a). At 0h after burn, the results of fluorescein staining of the 3 groups of mice showed that the 3 groups of mice had significant corneal epithelial defects, suggesting that the modeling of the 3 groups of mice was successful. At 24 and 48 hours, corneal epithelial defects were reduced in all three groups, but fluorescence staining was larger in the untreated and carrier groups than in the SSOE-treated group (FIG. 3c, d), indicating that SSOE treatment promoted healing of the corneal epithelium. On day 5 after burn, there was no staining in any of the three groups (Figure 3b), indicating healing of the corneal epithelium.
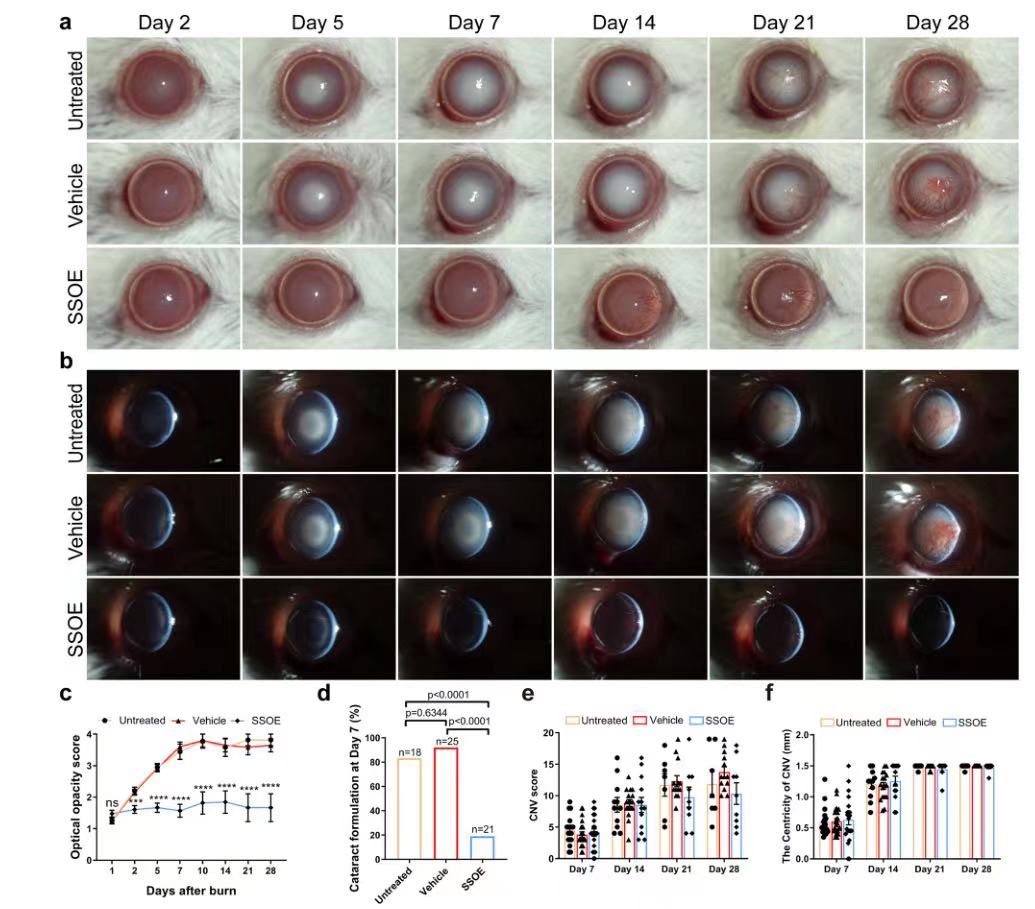
After alkali burn, the optical opacity gradually increased in the untreated and carrier treatment groups due to the aggravation of corneal opacity and cataract (FIG. 4a, b), while the optical opacity did not change significantly in the SSOE treatment group (FIG. 4c, P<0.0001), indicating that SSOE treatment reduced corneal opacity after alkali burn. Slit lamp examination and photography showed that the main cause of cataract in mice was nucleosclerosis of the lens. On the 7th day, the incidence of cataract in the untreated group and the carrier treated group was 83.3% and 92.0%, respectively, significantly higher than 19.0% in the SSOE treated group (Figure 4d, P<0.0001). Of the 21 SSOE-treated eyes, 17 did not develop significant cataracts during the 28-day study. In terms of anti-neovascularization, there was no significant difference between the three groups in either neovascularization score or vascular centrality (Figure 4e, f), suggesting that SSOE treatment had limited effect in reducing corneal neovascularization.
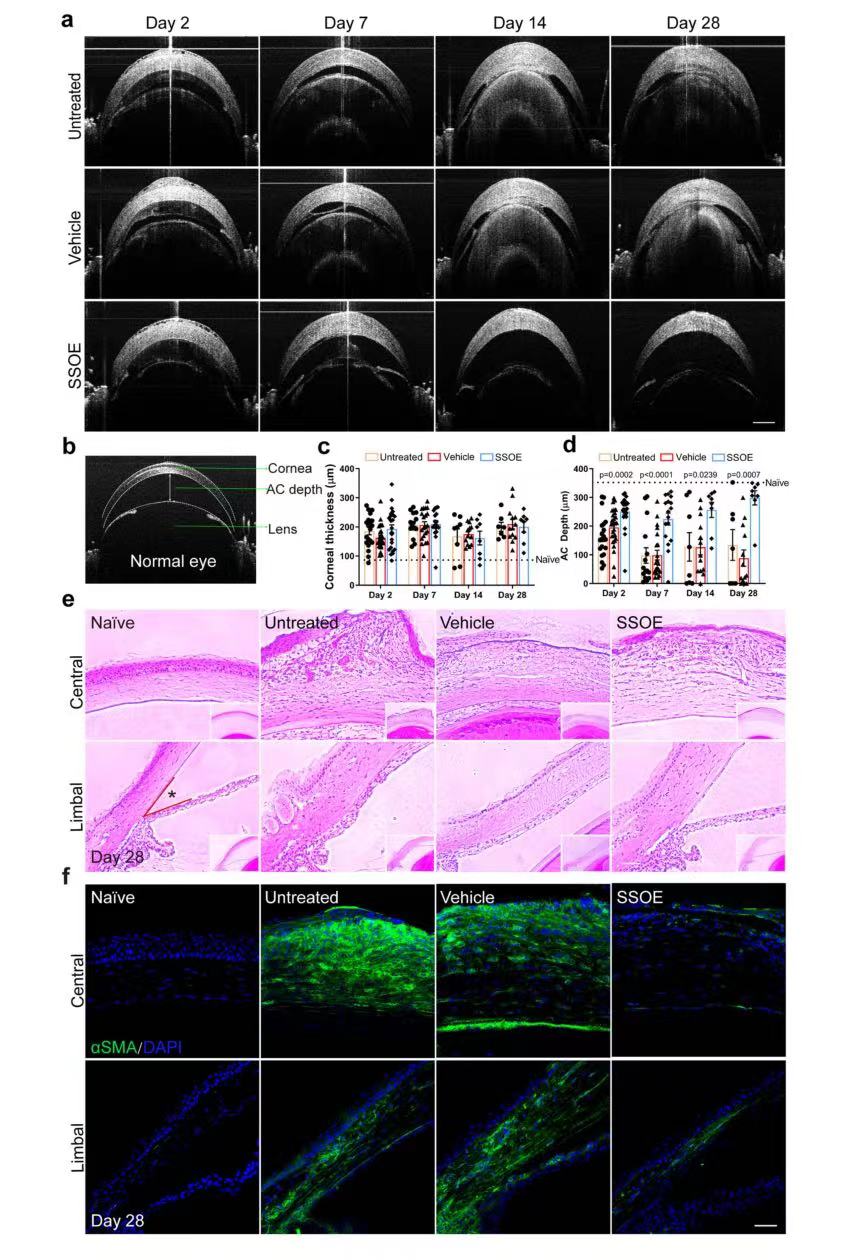
To investigate the effects of SSOE on ocular microanatomy and tissue integrity after alkali burn, continuous OCT imaging was performed (Figure 5a). It can be seen from the images that caustic soda burn leads to continuous corneal edema in mice, which is manifested as increased corneal thickness and massive exudation in the anterior chamber, followed by narrowing of the anterior chamber, and adhesion of iris/lens to the cornea in severe cases (FIG. 5a). There is no significant difference between the three groups in the treatment of corneal edema. However, after SSOE treatment, the anterior chamber depth (300 μm) remained close to that of normal mice (350 μm) (Figure 5d), which was significantly better than that of the other two groups. H&E staining (Figure 5e) revealed extensive leukocyte infiltration, tissue fibrosis, and corneal deformation in untreated and carrier treated eyes on day 28 after burn. In SSOE-treated eyes, inflammatory cell infiltration was reduced, corneal stroma disturbances were less severe (Figure 5e), and anterior chamber angles were close to normal (Figure 5e). The myofibroblast marker (α-SMA) indicates tissue fibrosis and scarring. Under normal conditions, α-SMA staining in the front of the eye, including the cornea, should be negative, whereas in untreated and carrier-treated eyes after alkali burn, α-SMA staining was evident throughout the cornea and part of the iris (FIG. 5f), whereas SSOE treatment resulted in reduced α-SMA staining. These results suggest that SSOE can reduce ocular inflammation and preserve tissue integrity after alkali burn.
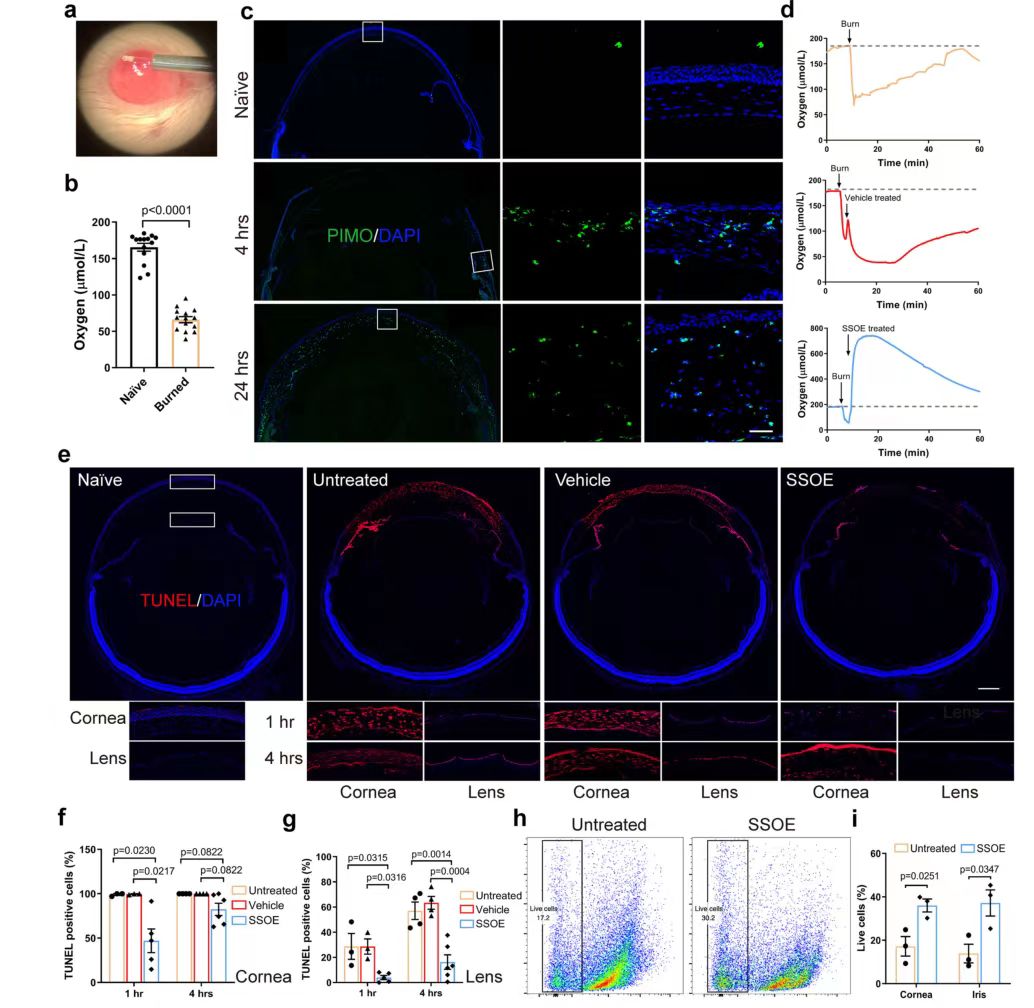
Using a miniature oxygen sensor (FIG. 6a), the researchers found that the oxygen concentration in the anterior chamber dramatically decreased by about 60% after alkali burn (P<0.0001, FIG. 6b), then gradually returned to the normal range within 50 minutes (FIG. 6d), and that SSOE treatment resulted in a rapid increase in oxygen concentration. A peak level of 534.8±164.7 μmol/L was reached within a few seconds and remained above baseline for more than 60 minutes (Figure 6d). Hypoxia of cells in the limbal region after alkali burn was observed by staining with the hypoxia marker PIMO (green staining) (Figure 6c), and hypoxia was present throughout the corneal cells at 24 hours. Hypoxia leads to cell death, so TUNEL staining was used to detect cell death after alkali injury (Figure 6E-G). At 1 hour after burn, almost all cells in the central cornea of untreated or carrier treated eyes were dead cells (TUNEL-positive), and in SSOE-treated eyes, TUNEL-positive cells were mainly present in the superficial upper cortex in direct contact with NaOH. At the same time, less TUNEL staining was observed in the iris of SSOE-treated eyes compared to untreated and carrier treated eyes. At 4 hours, the lens epithelial cells lining the anterior capsule of the lens were positive for TUNEL staining in untreated and carrier treated eyes, while there was almost no TUNEL positive in the lenses of SSOE-treated eyes. To verify these results, flow cytometry was also performed (FIG. 6h, i) and it was found that the percentage of live cells in the cornea treated with SSOE (36.0±3.0%) was significantly higher than in the untreated eyes 1 hour after burn (17.2±4.5%).
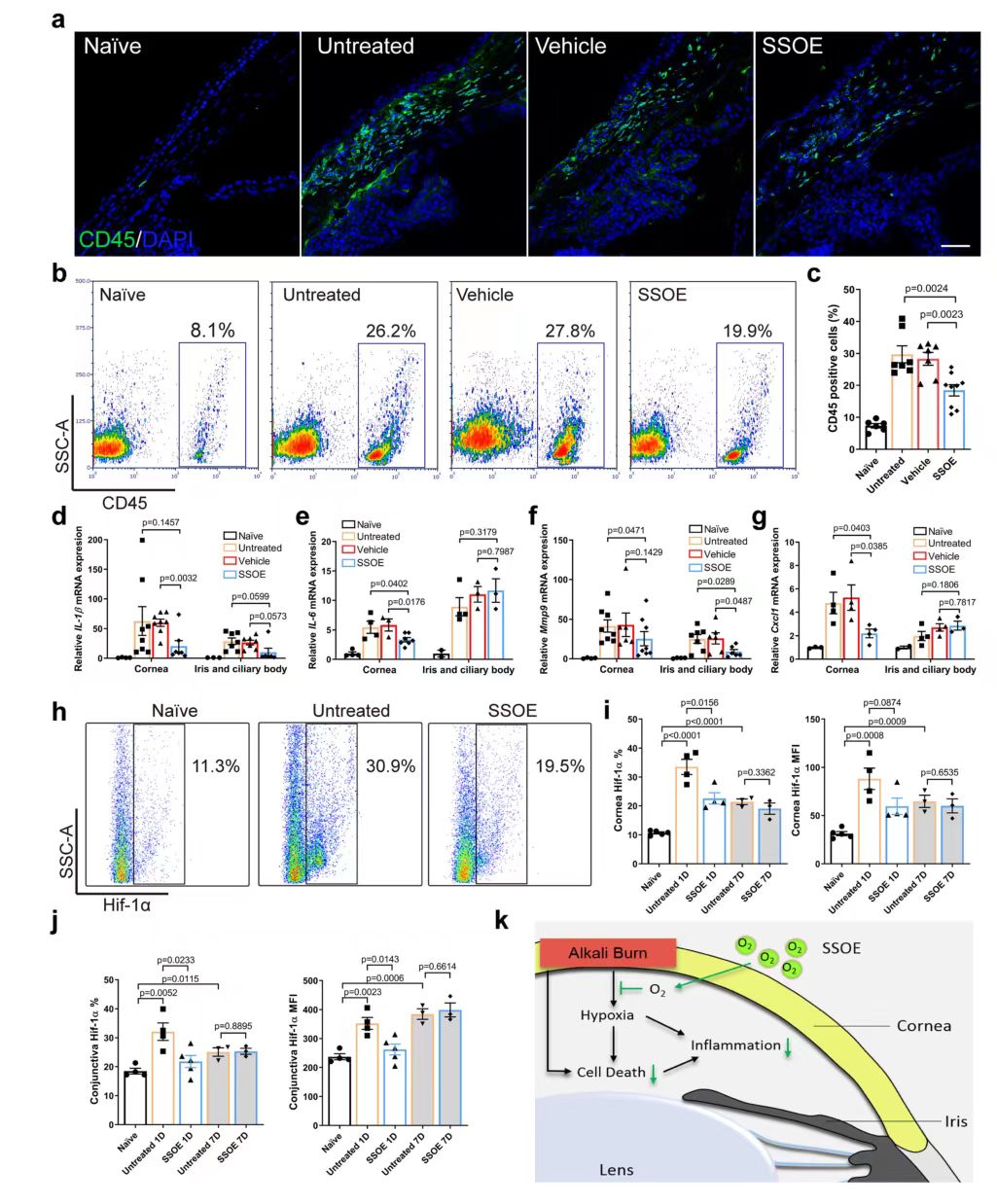
Finally, the cellular and molecular mechanisms of SSOE treatment were investigated. Immunohistochemical staining and flow cytometry results showed that the infiltration of CD45+ cells was significantly reduced after SSOE treatment (FIG. 7a, b, c). After burn, the expressions of inflammatory cytokines IL-1β and IL-6, Mmp9 and chemokine Cxcl1 in the cornea and iris/ciliary body were increased (Figure 7D-g), and SSOE significantly decreased their expressions in the cornea, as well as the expression levels of IL-1β and Mmp9 in the iris and ciliary body. Hypoxia-inducible factor (Hif) transcription factor is the primary regulator of tissue and cell response to hypoxia. Flow cytometry was used to determine HIF-1α (HIF-1A) expression levels (FIG. 7H-J). The percentage of Hif-1α positive cells and their protein levels in the cornea were significantly lower in the SSOE group than in the untreated group (FIG. 7i), and similar changes in Hif-1α signaling were also observed in the conjunctiva (FIG. 7j), suggesting that SSOE reversed tissue hypoxia after alkali burn.
Summary and discussion
Taking advantage of the beneficial role of oxygen in burn treatment, the study developed an oxygen emulsion for topical use in the eye that can be stored at room temperature for up to 1 year and maintains oxygen release capacity, which can be quickly and sustainably released without the need for additional tools to be directly targeted and distributed to the injured site when used. The emulsion is compatible with ocular surface cells and has no ocular toxicity to live animals. It can significantly reduce tissue hypoxia, inflammation, cell death and Hif-1α signaling after alkali burn, thus reducing the occurrence and healing of corneal opacity and cataract, and accelerating the healing of corneal epithelial wounds after alkali burn. This provides a useful idea for the treatment of acute alkali burn.